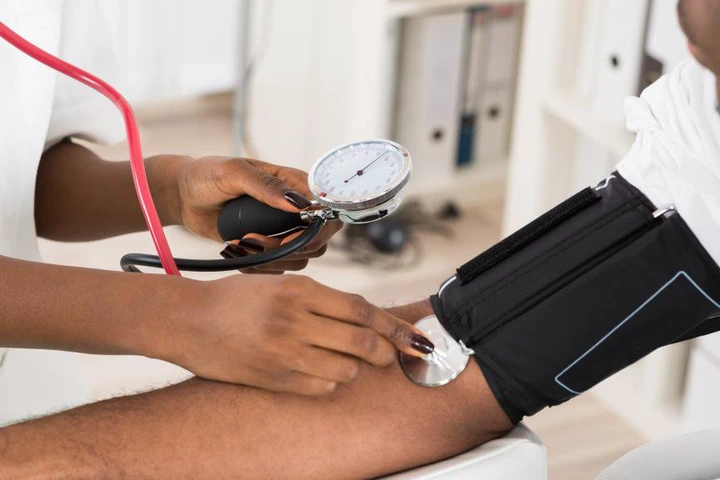
A novel low-dose 3-in-1 blood pressure pill has significantly outperformed standard care, a research has shown.... CLICK TO READ THE FULL NEWS HERE▶▶
According to the research, over 80 percent of patients achieved control within a month, sustained at six months.
The research shows that a treatment plan based on novel combination of low doses of three anti-hypertensive drugs in a single pill – known as GMRx2 – was superior to a high-quality standard care treatment plan at lowering blood pressure in patients with uncontrolled hypertension.
Results of the trial which was led by the George Institute for Global Health, termed ‘deliVERy of Optimal blood pressure coNtrol in afrICA (VERONICA)-Nigeria’ were presented at the European Society of Cardiology Congress 2024 and simultaneously published in the Journal of the American Medical Association (JAMA) on August 31, 2024.

“The GMRx2 treatment plan involved a once daily pill containing telmisartan, amlodipine and indapamide at a quarter, half or standard doses.
“The standard care treatment plan recommended by the Nigerian Ministry of Health began with monotherapy, followed by dual and triple combination therapy, and was typical of hypertension guidelines for many countries.
“After 6 months treatment, home systolic blood pressure was 31mmHg lower in the GMRx2 group compared to 26 mmHg lower with standard care – the 5.8 mmHg difference was highly clinically and statistically significant. Existing evidence shows that with every 5 mmHg reduction in systolic blood pressure there is a 10 percent reduction in major cardiovascular events such as stroke, heart attack and heart failure,” the research noted.
It added that after just one month, 81 percent of participants in the GMRx2 group achieved clinic-measured blood pressure control versus 55 percent with standard care.
“This improvement was sustained at six months with 82 percent achieving control, compared with 72 percent under standard care. Tolerability of both treatment plans was good, with no withdrawals due to adverse events,” the research explained.
Speaking on the research, Head of the Cardiovascular Research Unit at the University of Abuja, Nigeria and study principal investigator, Prof Dike Ojji expressed excitement over the new findings.
“The triple pill still produced clinically meaningful reductions in blood pressure compared to standard care, even when standard care closely followed current guidelines and involved more clinic visits.
“In low-income countries fewer than one in four treated people achieve blood pressure control and in high-income settings it is only between 50 percent and 70 percent ,” he said
Ojji added that to see rates of over 80 percent control on patients in just one month was impressive.
It is estimated that over a billion adults live with hypertension worldwide, with two-thirds living in low- and middle-income countries.
High blood pressure is the leading risk factor for mortality, accounting for 10.8 million deaths a year and researchers hoped this new treatment could have a big impact on reducing rates of cardiovascular disease, particularly in countries with the highest burden.
Also speaking on the issue, Senior Professorial Fellow at The George Institute and Chief Medical Officer at George Medicines, Prof. Anthony Rodgers, explained the institution’s mission is to develop sustainable solutions that can improve the health of millions of people worldwide and alleviate strain on health systems.
“ There is a global goal to reach 80 percent blood pressure control among those treated, but no country has yet achieved this. With the VERONICA trial, we’ve shown the potential of this novel strategy to reach this ambitious target.
“There has been little innovation in this field, so it’s rewarding to see many years of research by The George Institute culminate in a novel treatment using established medicines to address an unmet need,” he added.
The VERONICA trial is funded by the Australian National Health and Medical Research Council.